How Can You Increase the Solubility of Sugar in Water
Calcium hydroxide is Retrograde soluble meaning its solubility increases with lowering of temperature. At 0 Deg C 20 Deg C and 100 Deg C.
How can you increase the solubility of sugar in water for Class 6.
. If sugar is added to water it just becomes liquid sugar - httpsinside-nescafeweebly. So it will affect the solubility of sugar in water. The solubility of a solute increases on heating the solution.
The solubility of sucrose in water increases as the temperature increases. Solubility increases by adding more solvent. Which factor would not affect the solubility of.
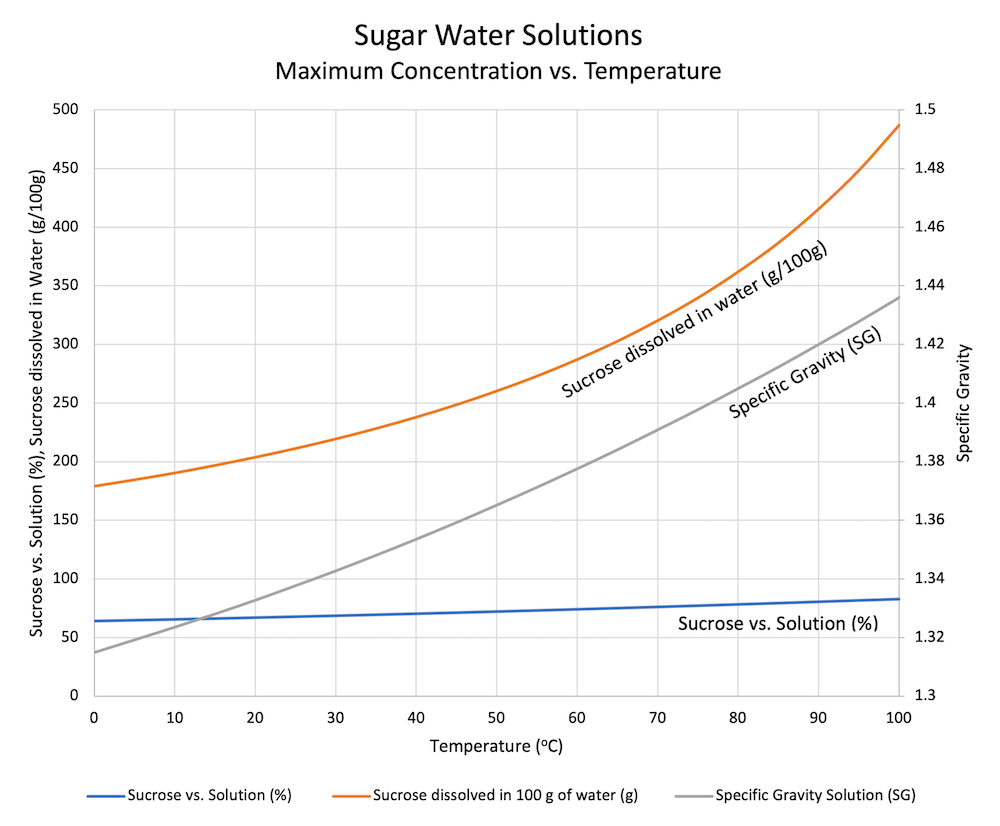
A natural emulsifier would be the Lecithin. What is one way to. 20 g100 g water at 20ºC 280 g100 g water at 80ºC and so forth.
Basically solubility increases with temperature. Increase the surface area of the sugar. Stirling the water and sugar will increase the solubility of sugar in water.
Solubility is a thermodynamic property that. The solubility of sugar or sucrose in water varies with temperature ranging from 179 grams per 100 milliliters at 20 degrees Celsius to 487 grams per 100 milliliters at 100. Decrease the amount of water.
How can the solubility of sugar be increased in water. Bincreasing the surface area of the solute. Similarly you may ask what are 3 factors that affect solubility.
Three ways I can come up with are increasing the temperature increased the amount of solvent and using a solvent with similar polarity as the solute. The solubility of a solute can be increased by the following ways. 22 rows Herrington found that the addition of calcium chloride to a lactose solution increased the solubility of lactose from 286 to 295 grams per 100 grams of water at 32C.
For example more sugar can be dissolved in hot water than in cold water. The easiest way is to increase the temperature of the solution use hot waterThere are also other ways. If two unidentified solids of the same texture and color have different solubilities in 100 grams of water at 20 degrees Celsius you can conclude that.
Increase the temperature of the water. For example if mixing sugar and water you would add more water to increase solubility. Cincreasing the pressure of the solution.
What is one way to increase the solubility of sugar in water. Sugar is a carbohydrate therefore wearing covalent bonds. When we grind the sugar to make it finer the sugar will.
Aincreasing the temperature of the solvent. When the maximum amount of a substance is dissolved in water. Increase the amount of sugar.
Look up more places. The solution is said to be saturated. Just cool the solution.
Stirring will dissolve more solute in a solvent. Adding more solute to the solution will just cause some of the solute to precipitate. As water temperature increases the amount of sugar that will.
Dshaking or stirring the solution. That is the substance that the other is being dissolved into. At room temperature roughly 20 degrees C you can dissolve 2039 grams or 200 grams of sugar in 100 mL of water.
How Much Sugar Can Be Dissolved In 250g Of Water At 80 C Quora

Comments
Post a Comment